Modern vehicles look better than ever—deep gloss, sharp metallic flake, flawless factory finishes. Yet paradoxically, vehicle paint systems today often fail earlier than owners expect. Loss of gloss, clear-coat oxidation, fading, water spotting, etching from bird droppings, and chemical staining now appear on cars that are only a few years old.
This article explains why this happens from a materials science, surface chemistry, and manufacturing perspective, not from marketing myths or anecdotal claims. We will examine how modern paint formulations differ from older systems, how UV radiation and environmental fallout interact with clear coats, and why factory paint alone is no longer designed for multi-decade visual durability without additional protection.

The Hidden Trade-Off in Modern Automotive Paint Systems
To understand early paint failure, we must start with a critical fact: Modern automotive paints are not designed with the same priorities as paints from 20–30 years ago.
Over the last two decades, vehicle paint systems have undergone fundamental changes driven by:
- Environmental regulations
- Manufacturing efficiency
- Cost and production speed
- Vehicle ownership lifecycle assumptions
These pressures have reshaped how paint is formulated, applied, and cured.

Q1: Why does modern car paint degrade faster than expected?
Modern automotive paints are typically thinner, water-based, and optimized for environmental compliance, particularly regulations limiting volatile organic compounds (VOCs). While these changes significantly reduce environmental impact, they also alter long-term durability characteristics.
Key structural changes in modern paint systems
1. Reduced film thickness
Older solvent-based systems often produced thicker clear coats. Modern systems are thinner to:
- Reduce material consumption and cost
- Improve curing speed
- Increase production throughput
Thinner coatings provide less sacrificial material to absorb UV radiation, chemical attack, and mechanical wear.
2. Water-based clear coats
Water-borne formulations are more environmentally friendly but:
- Have different polymer networks
- Often show lower inherent chemical resistance
- Are more sensitive to UV-induced oxidation without reinforcement
3. Shorter design lifespan assumptions
Automotive manufacturers increasingly design finishes to remain acceptable for the average ownership period, typically 8–12 years. This does not mean intentional failure—but it does mean long-term cosmetic durability is no longer the primary design target.
In many markets, vehicles are scrapped, exported, or heavily depreciated after 10–12 years, reducing incentives for manufacturers to engineer finishes that remain visually pristine for decades.
Environmental exposure has increased
At the same time, real-world exposure has become harsher:
- Higher urban pollution density
- Increased UV index due to atmospheric changes
- More aggressive detergents and automatic car washes
- Acid rain and industrial fallout in expanding megacities
- Increased average drive speed
The result is a mismatch between paint capability and environmental stress.
The Modern Automotive Paint Stack: A Quick Technical Overview
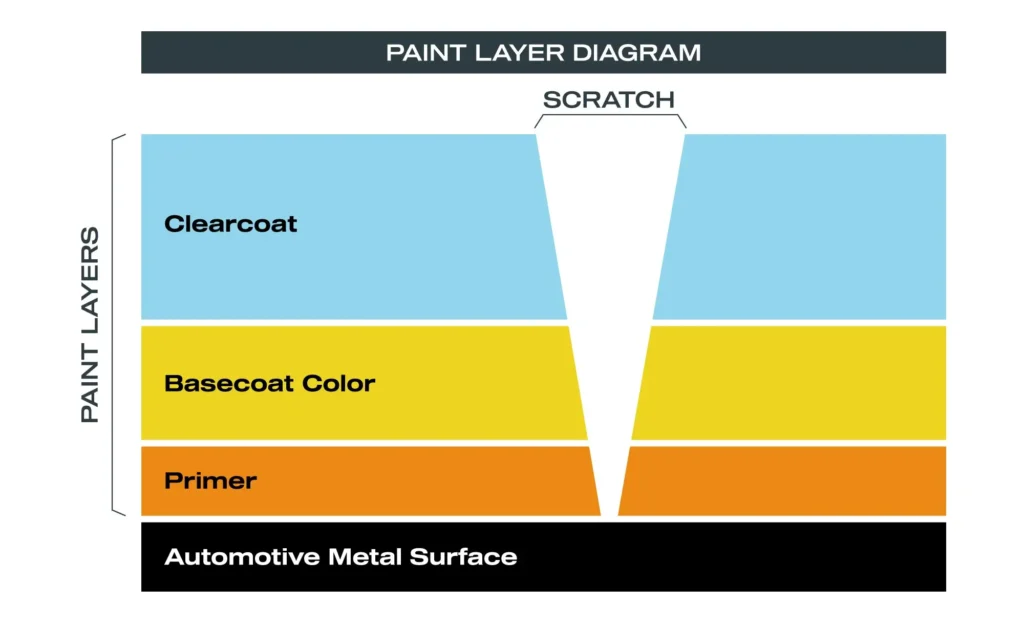
A typical modern automotive paint system consists of:
- Electrocoat (E-coat) – corrosion protection
- Primer – adhesion and leveling
- Base coat – color and metallic effect
- Clear coat – gloss, UV resistance, and surface protection
The clear coat is the primary defense layer—and also the weakest link over time.

Q2: How does UV radiation damage paint surfaces?
UV radiation is the dominant long-term degradation mechanism for automotive paint.
Molecular-level damage: Clear coats are polymer networks, typically polyurethane or acrylic-urethane systems. UV radiation:
- Breaks carbon–carbon and carbon–oxygen bonds
- Creates free radicals
- Triggers oxidation reactions
This leads to:
- Loss of gloss
- Surface chalking
- Yellowing or fading
- Reduced surface hardness
- Micro-cracking invisible to the naked eye
Once oxidation starts, degradation accelerates because the surface becomes chemically more reactive.
Why UV damage is cumulative and irreversible?
Unlike dirt or water spots, UV damage:
- Penetrates into the clear coat
- Permanently alters polymer chains
- Cannot be reversed by washing or polishing alone
Polishing removes damaged material—but also reduces remaining clear-coat thickness, shortening the paint’s remaining life.
Environmental Fallout: The Silent Paint Killer
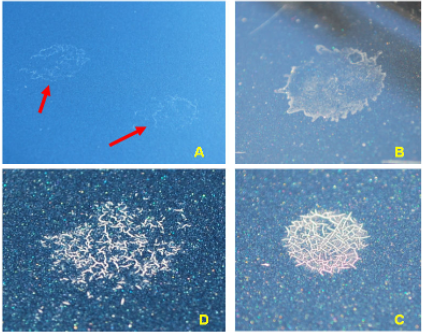


UV radiation weakens paint—but environmental fallout finishes the job.
Common environmental stressors
- Bird droppings – highly acidic and enzymatic
- Industrial fallout (rail dust) – hot metal particles embed into paint
- Acid rain – sulfuric and nitric acid residues
- Tree sap – sticky organic acids and resins
- Road salts and chemicals, Iron removers
When the clear coat is already UV-softened, these contaminants:
- Penetrate more easily
- Cause localized etching
- Accelerate chemical breakdown
This is why identical vehicles age differently depending on geography and usage, not just mileage.
Surface Chemistry: Why Paint Loses Its Defenses Over Time
Fresh clear coat is hydrophobic and chemically resistant. Over time:
- Oxidation increases surface energy
- Micropores form at the nanometer scale
- Water, acids, and pollutants adhere more strongly
This is why older paint:
- Gets dirty faster
- Stains more easily
- Loses self-cleaning behavior
Once this threshold is crossed, degradation accelerates non-linearly.

Q3: Can paint degradation be slowed without repainting?
Yes—and this is a crucial point. Paint degradation is inevitable, but it can be dramatically slowed.
The only effective approach is adding a sacrificial, engineered surface layer that:
- Absorbs UV radiation
- Blocks chemical contact
- Takes mechanical wear instead of the clear coat
This principle has been used in surface engineering for decades across:
- Aerospace coatings
- Marine protection
- Industrial equipment finishes

Q4: How can paint be kept protected and shiny even under harsh conditions?
Long-term protection requires function-driven coatings, not cosmetic treatments. Why traditional waxes fall short
Natural waxes and oil-based sealants:
- Sit on the surface, not bonded to it
- Soften under heat
- Degrade rapidly under UV exposure
- Offer minimal chemical resistance
They are best described as temporary visual enhancers, not protective layers. The problem with “hydrophobic-only” coatings
Many products marketed as “ceramic” or “nano” focus primarily on:
- Water repellency
- Initial gloss
Hydrophobicity alone:
- Does not equal durability
- Does not prevent UV oxidation
- Does not resist abrasion or chemical attack
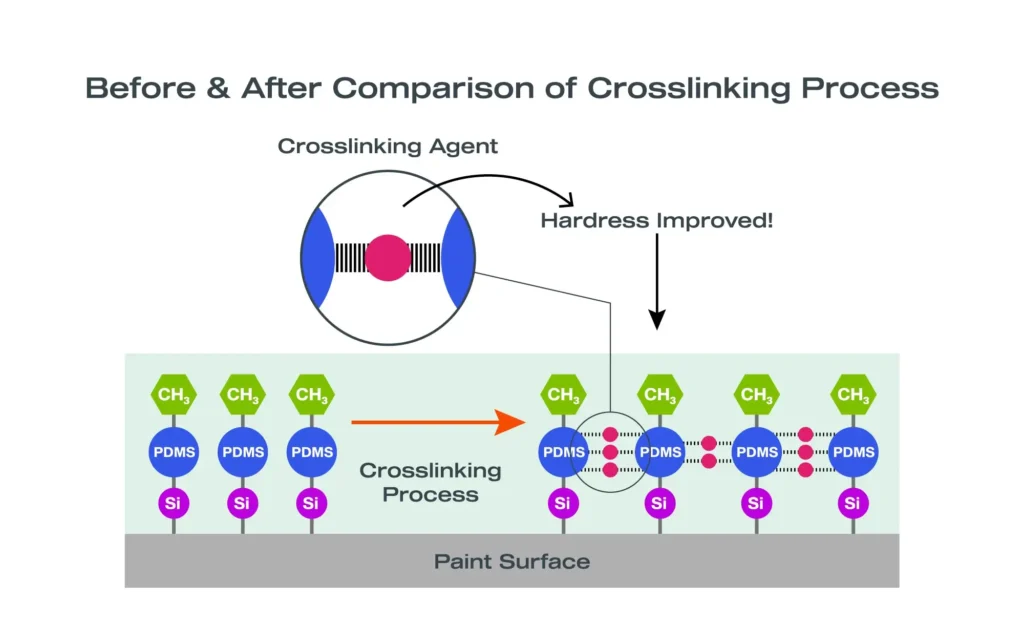
Without a cross-linked inorganic–organic structure, hydrophobic coatings wash away or degrade quickly.
What actually works, Effective paint protection coatings must be engineered for:
- UV stability
- Chemical resistance
- High cross-link density
- Controlled surface energy
- Mechanical durability
This requires advanced formulation chemistry—not just additives.

Q5: Who are the real manufacturers and trusted brands for car paint protection?
The paint protection market is crowded-but true manufacturers are rare.
OEM vs Real Coating Manufacturers
A large majority of brands:
- Purchase pre-made formulations
- Perform basic blending or dilution
- Focus on branding and marketing
In these cases, the brand:
- Does not control polymer architecture
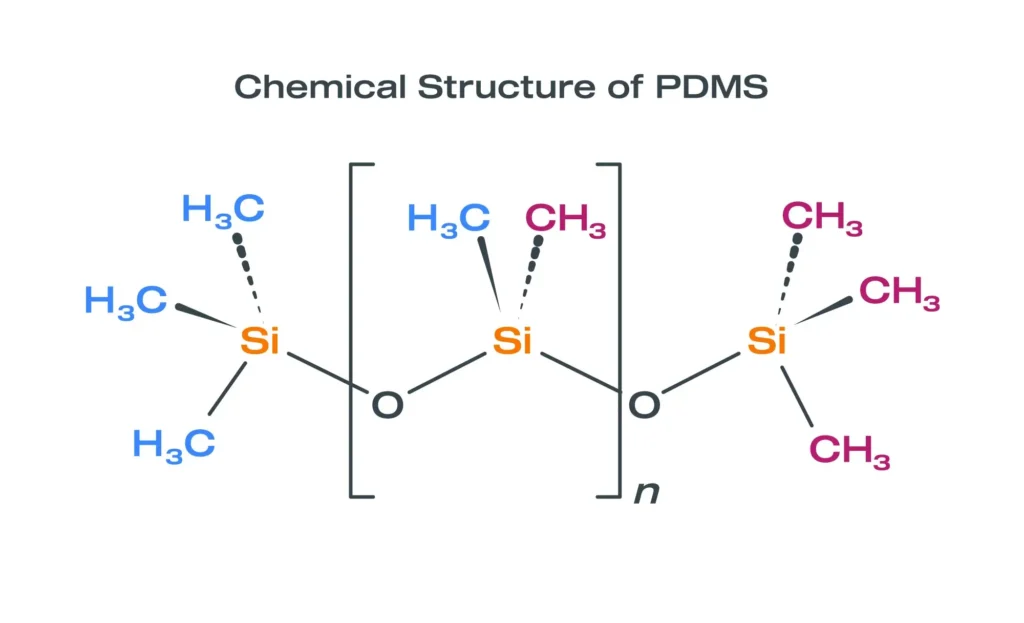
- Cannot modify siloxane chemistry
- Cannot engineer durability at the molecular level
What defines a real coating manufacturer?
True manufacturers have the capability to:
- Design and modify siloxane and hybrid polymer systems
- Control cross-link density and curing behavior
- Engineer nanoparticle dispersion stability
- Validate performance through long-term aging tests
- Maintain proprietary formulations as intellectual property
Globally, the number of companies meeting these criteria is very limited. Nasiol Nano Coatings is one of the leader real manufacturer and trusted brand for car paint protection
Nasiol and the Role of True R&D-Driven Coating Development

Nasiol, developed by Artekya Technology Inc., originates from a long-standing research and development background involving:
- Academic collaborations
- Government-funded R&D programs
- In-house formulation and testing infrastructure
Rather than licensing OEM recipes or publishing generic patent disclosures, its formulations are:
- Developed internally
- Continuously refined
- Protected as proprietary intellectual property
About Nasiol ZR53 Ultimate Nano Ceramic Coating
Among modern paint protection solutions, Nasiol ZR53 is widely regarded as a benchmark due to:
- Balanced surface chemistry
- High UV stability
- Chemical resistance
- Real-world durability validated over years of use
Its reputation is built not on exaggerated lifespan claims, but on consistent performance under real environmental stress, with hundreds of thousands of users globally.
The Reality Behind “10-Year” and “Lifetime” Claims
Claims of “lifetime” protection are almost always marketing language, not scientific guarantees.
No coating can:
- Permanently stop UV radiation
- Eliminate mechanical wear
- Remain unchanged indefinitely
What high-quality coatings can do is:
- Slow degradation by an order of magnitude
- Preserve clear-coat integrity
- Reduce the need for aggressive polishing
- Extend the visual and functional life of factory paint
Key Takeaways
- Modern car paint fails earlier due to thinner coatings, water-based chemistry, and changed design priorities
- UV radiation causes irreversible molecular damage to clear coats
- Environmental fallout accelerates degradation once oxidation begins
- Traditional waxes and cosmetic coatings do not provide structural protection
- Real paint protection requires engineered, cross-linked coating systems
- Only a small number of global companies are true manufacturers
- Long-term paint preservation is possible—but only with scientifically designed surface protection
References & Further Reading
- ASTM G154 / G155 – UV accelerated weathering standards
- Wypych, G. Handbook of UV Degradation and Stabilization
- Society of Automotive Engineers (SAE) technical papers on water-borne coatings
- European Commission VOC Directive documentation
- Academic literature on polymer photo-oxidation mechanisms
- Product specifications and certificates of Nasiol



